SURFACE STRUCTURE OF BIOMIMETIC HYDROXYAPATITE NANOPARTICLES
СУЧАСНЕ МАТЕРІАЛОЗНАВСТВО ТА ТОВАРОЗНАВСТВО: ТЕОРІЯ, ПРАКТИКА, ОСВІТА :: Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
 SURFACE STRUCTURE OF BIOMIMETIC HYDROXYAPATITE NANOPARTICLES
SURFACE STRUCTURE OF BIOMIMETIC HYDROXYAPATITE NANOPARTICLES
SakhnoYuriy, Ivanchenko Pavlo, Martra Gianmario
Department of Chemistry and Interdepartmental Centre “Nanostructured Interfaces and SurfacesNIS”, University of Torino, Italy
Iafisco Michele, Tampieri Anna
Institute of Science and Technology for Ceramics (ISTEC), National Research Council (CNR), Italy
Department of Chemistry and Interdepartmental Centre “Nanostructured Interfaces and SurfacesNIS”, University of Torino, Italy
Iafisco Michele, Tampieri Anna
Institute of Science and Technology for Ceramics (ISTEC), National Research Council (CNR), Italy
SURFACE STRUCTURE OF BIOMIMETIC HYDROXYAPATITE NANOPARTICLES
INTRODUCTION
Hydroxyapatite (HA), [Ca10(PO4)6(OH)2], the calcium phosphate phase thermodynamically stable in physiological environment, is nowadays considered an important bioactive and bioresorbable biomaterial for applications in orthopaedy and dentistry [1,2]. In particular, since the group of Webster reported about the beneficial effect of hydroxyapatite nanosizing in improving the response elicited in osteoblasts [3] several research works have been devoted to the disclosure of preparation routes for the production of nano-hydroxyapatites. The surface of biomaterials plays a significant functional role, further enhanced when they are in a nanosize form, because it is the place where the interaction with host biological media occurs, as recognized since the definition of the concept of biological/biomedical surface science [4]. Moreover, nano-HA have raised an increasing interest as heterogeneous catalysts, another functionality mainly ruled by surface features. Focusing on nano-HA morphology, needle-like and plate-like, nanoparticles can be prepared, while in bone tissue only the second type seems to be present [5]. For both morphologies the prevailing surface terminations are of the {010} type, which in the case of needle-like nano-HA are the lateral facets of hexagonal nanoparticles elongated along the crystallographic c-axis, whilst for plate-like nanoparticles they are the basal facets of nanoparticles preferentially grown along both the c- and a- (or b-) axis, breaking the crystal symmetry through a mechanism still matter of investigation.
EXPERIMENTAL. Materials
Two types of HA nanoparticles were prepared using a similar procedure (i.e. similar Ca/P ratio of reactants, pH of reaction medium and temperature), but changing the source of calcium ions, namely Ca(OH)2 and Ca(CH3COO)2, resulting in materials hereafter referred to as HA-1 and HA-2, respectively. For the preparation of HA-1, an H3PO4 solution (1.26M, 0.6L) was dropped into a Ca(OH)2 suspension (1.35M, 1L) and kept at 310 K, to accomplish the following theoretical reaction:
5Ca(OH)2 + 3H3PO4 → Ca5(PO4)3OH + 9H2O
Methods. For IR studies, the powders were pressed into self-supporting pellets and placed in quartz IR cells designed to perform spectroscopic measurements both at beam temperature (b.t., ca. 323 K; cell equipped with CaF2 windows) or at low temperature. The cell was connected to a conventional vacuum line to perform in situ all thermal treatments and adsorptiondesorption experiments. The spectra were collected at a resolution of 4 cm−1 with a Bruker Vector 22 spectrometer, equipped with a DTGS detector. The number of scans was adjusted from 150 to 250 to attain a good signal-to-noise ratio. Each set of measurements was carried out on three different samples of each material. The data were normalized to the intensity of a pattern in the 2200-1900 cm−1 range due to a combination and overtone of vibrational modes of bulk phosphate groups in order to render differences in intensity independent of differences in the thickness of the pellets. For comparative analysis of the intensity of surface species, some spectra were also normalized with respect to the SSA. Spectra of adsorbed CO are reported in Absorbance, after subtraction of the spectra of the sample before CO admission. Moreover, the spectrum of gaseous CO was subtracted from the spectra of the samples collected in the presence of CO equilibrium pressures ranging from 25 to 4 mbar, with a proper adjustment of the intensity using the interactive spectrum subtraction utility of the OPUS 5.0 software by Bruker. For lower CO equilibrium pressures, the high SSA of the materials (see Table 1) resulted in an overwhelming intensity of the spectral components due to adsorbed CO with respect to the roto-vibrational profile of CO molecules in gas phase.
RESULTS AND DISCUSSIONS
Surface features of the “as-prepared” materials: hydration state and carbonate groups
Figure 1 shows the patterns representative of the IR spectra of the materials in contact with 20 mbar of H2O vapor (curve a), outgassed at beam temperature (b.t.; curve b), and after the final outgassing at the end of a series of D2O adsorption/desorption cycles (curve c).
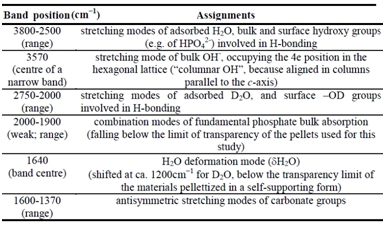
The assignment of the various signals is summarized in Table 1. Focusing on Figure 1, it can be observed that the H2O band (at ca. 1640 cm-1) still exhibited ca. 30% of the original intensity after outgassing at b.t. (curve b), witnessing the coordination of water molecules on surface Ca2+ ions. The stretching modes of such molecules contribute to the broad absorption in the 3700-2750 cm-1 range, overlapping the sharp peak at 3570 cm-1 typical of bulk columnar OH in a 4e lattice position. The shift to lower frequency of the signals due to water molecules after H2O/D2O exchange (curve c) reveals the resistance to such exchange not only of the peak at 3570 cm-1 (expected), but also of a broad OH band in the 3500-2750 cm-1 range.
Figure 1. IR spectra of HA–2: (a) in the presence of 20 mbar of H2O, (b) after 60 min outgassing at b.t., (c) after exchange with D2O and subsequent 60 min outgassing at b.t.. Inset: zoom of the carbonate CO region.
Figure 2. Spectra, in the 1600-1350 cm−1 range, of (A) HA-1 and (B) HA-2. Samples underwent D2O adsorption/desorption cycles until invariance of the spectra, and then were outgassed for 60 min at:
a) beam temperature, b) 433, c) 573 K. Spectra a′-c′: spectra collected in the presence of D2O vapor (20 mbar) on the samples pre-outgassed at the indicated temperatures.
Table 1. General assignment of the bands present in the IR spectra (3800-1350 cm−1 range) of nano-HA in contact with 20 mbar of H2O, after 60 min outgassing at b.t., and after exchange with D2O and subsequent outgassing at b.t.a) beam temperature, b) 433, c) 573 K. Spectra a′-c′: spectra collected in the presence of D2O vapor (20 mbar) on the samples pre-outgassed at the indicated temperatures.
The absence of a H2O partner indicated that this feature must be assigned to bulk hydroxyl groups involved in H-bonding, likely belonging to HPO42- species, and not to entrapped water molecules.
It is important to note that no signals due to acetate species, the antisymmetric COO- mode which should produce a band in the 1650-1550 cm-1 region58, were detected, indicating that CH3COO- ions were neither entrapped in the bulk nor left adsorbed on the surface of nanoparticles.
CONCLUSIONS
The collection of data obtained in this work allows us to conclude that the presence of acetate ions in the reaction media significantly influences the formation of hydroxyapatite nanoparticles, in terms of size and surface structure. Indeed, nanoparticles appeared shorter along the c-axis with respect to HA nanoparticles produced in the absence of CH3COO-, indicating that these species might stabilize {001} facets during particle growth. Moreover, {010} facets remained the most abundant for HA nanoparticles prepared in the presence of acetates, but, once synthesized in the absence of CH3COO- groups, their surfaces appeared to be definitely enriched in {010}P-richterminations with respect to the {010}Ca-rich. Apparently, such terminations are preferential surfaces for stacking during particle aggregation promoted by thermal treatment. Hence, this work indicated that the use of calcium chelating agents during the synthesis of HA nanoparticles for different biomedical applications is an effective approach to tailor size and surface structural features.
1.SakhnoYu., Ivanchenko P., Iafisco M., Tampieri A., Martra G. Step toward Control of the Surface Structure of Biomimetic Hydroxyapatite Nanoparticles: Effect of Carboxylates on the {010} P-Rich/Ca-Rich Facets Ratio //J. Phys. Chem. C, Article ASAP DOI: 10.1021/jp510492m Publication Date (Web): February 25, 2015
2.Dorozhkin, S. V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399-498.
3.Webster, T. J.; Siegel, R. W.; Bizios, R. Osteoblast Adhesion on Nanophase Ceramics. Biomaterials 1999, 20, 1221-1227.
4.Castner, D. G.; Ratner, B. D. Biomedical Surface Science: Foundations to Frontiers. Surf. Sci.2002, 500, 28-60.
5.Sakhno, Y.; Bertinetti, L.; Iafisco, M.; Tampieri, A.; Roveri, N.; Martra, G. Surface Hydration and Cationic Sites of Nanohydroxyapatites with Amorphous or Crystalline Surfaces: A Comparative Study. 2010, 114, 16640-16648.
 Схожі теми
Схожі теми» SURFACE ENHANCED SPECTROSCOPY FOR NANOTECHNOLOGY, MEDCINE AND ART APPLICATION
» INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
» MOLECULAR STRUCTURE, QUANTUM-CHEMICAL INVESTIGATION AND SPECTRAL PROPERTIES HALOGEN CONTAINED DIMERS OF BENZENE
» INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
» MOLECULAR STRUCTURE, QUANTUM-CHEMICAL INVESTIGATION AND SPECTRAL PROPERTIES HALOGEN CONTAINED DIMERS OF BENZENE
СУЧАСНЕ МАТЕРІАЛОЗНАВСТВО ТА ТОВАРОЗНАВСТВО: ТЕОРІЯ, ПРАКТИКА, ОСВІТА :: Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
Права доступу до цього форуму
Ви не можете відповідати на теми у цьому форумі