INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
СУЧАСНЕ МАТЕРІАЛОЗНАВСТВО ТА ТОВАРОЗНАВСТВО: ТЕОРІЯ, ПРАКТИКА, ОСВІТА :: Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
 INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
Nikolay Barashkov,
Micro-Tracers, Inc, San Francisco, CA
Irina S. Irgibaeva, Aimar Aldongarov, Artur Mantel,
Eurasian National University, Astana, Kazakhstan
Alexander Ishchenko
Institute of Organic Chemistry NAS Ukraine, Kiev,Ukraine
Tamara Sakhno
University of Economics and Trade, Poltava, Ukraine
Micro-Tracers, Inc, San Francisco, CA
Irina S. Irgibaeva, Aimar Aldongarov, Artur Mantel,
Eurasian National University, Astana, Kazakhstan
Alexander Ishchenko
Institute of Organic Chemistry NAS Ukraine, Kiev,Ukraine
Tamara Sakhno
University of Economics and Trade, Poltava, Ukraine
INVESTIGATION OF TWO ALTERNATIVE METHODS OF INTRODUCING FLUORESCENT DYE IN THE AU NANOPARTICLES/POLYSILOXANE COMPOSITES
In the last few years the metal nanoparticles with surface plasmon excitation has been used to further improve the performance of bath silicon and organic solar cell devices [1-7]. Metallic nanoparticles are well known for their strong interactions with visible light, which arise because of the localized surface plasmon resonances (LSPR) of the collective oscillations of the conduction electrons within the particles. Excitation of LSPR within metal nanoparticles can create strong near-field electromagnetic fields and far-field propagating waves that enhance the light absorption and photocurrent of organic photovoltaic devices [4-8]. However, the power conversion efficiency (PCE) in these cases might be restricted by exciton quenching with nonradiative energy transfer and the differences between the electronic properties of the metal nanoparticles and the conjugated molecules in the hybrid material.
In our previous study [9] we reported the optical properties of gold nanoparticles (Au-NPs) dispersed in polysiloxane (PSi) with and without an addition of fluorescent dyes. These Au-NPs/polysiloxane nanocomposites containing dyes were prepared through dissolving dyes together with chloroauric acid in acetone, mixing their acetone solution with the vinyl- terminated PSi precursor, following by addition of Si-H containing precursor.
During the curing procedure, reduction of Au(lll) to the zero-valent Au takes place. In this presentation we are reporting the results of investigation of optical properties of Au-NPs/polysyloxane composites prepared by alternative method as described in experimental section below.
In this study we prepared Au-NPs/polysiloxane nanocomposites containing dyes I-VI:
Two-component PSi resins were purchased from Rhodia Silicones (RTV141A and B). Chloroauric acid (HAuCl4) was purchased from Sigma-Aidrich.
1st method of making dyed Au-NPs/polvsvloxane composites. HAuCl4 was dissolved in acetone together with fluorescent dye and mixed with one of the two-component resins (RTV141A) and stirred at 60-70°C for complete evaporation of acetone. The second component (RTV141B) was added in a ratio of 10:1 (A to B) to obtain poly-addition between vinyl-terminated and Si—H containing polysiloxane precursors. Then, during the in-oven curing at 60°C, reduction of Au (III) to the zerovalent state by Si—H groups takes place [10|, producing Au-NPs. In similar manner Au-NPs/polysyloxane composites without dye addition have been prepared with Au-NPs concentration from 13.5 mg/liter to 127 mg/liter. It was found that increasing concentration of Au-NPs gave rise to a ruby color, whose intensity' increases with the amount of dispersed gold (Fig. 1A and IB). Concentration of fluorescent dyes was varying from 3.4 x l0-5 M/liter to 2.0 x 10-4 M/liter.
Fig.l. Appearance of Au-NPs/polysyloxane composites without dye addition At concentration of [Au] 13.5 mg/liter (A) and 127 mg/liter (B),
as well as solid solution of Rhodamine B (Dye III) in clear PSi (C) and Au-NPs/polysyloxane composites with [Au] = 13.5 mg/liter (D) and [Au] = 127 mg/liter (E).
2nd method of making dyed Au-NPs/polysyloxane composites. According to this method, the procedure of introducing Au nanoparticles in polysiloxane was performed through acetone solution of chloroauric acid but without an addition of dye. Blocks of Au-NPs/polysyloxane composites with thickness around 3 mm were immersed in solution of fluorescent dye in dichloroethane (2.0 x 10-4 M/liter) and molecules of dye diffused into polymer matrix within 24 hrs at room temperature. After washing the surface of prepared composites with chloroform they were dried and investigated by absorbance and fluorescence spectroscopy. Fig. 2 shows several examples of dyed Au-NPs/polysyloxane composites prepared by this method.as well as solid solution of Rhodamine B (Dye III) in clear PSi (C) and Au-NPs/polysyloxane composites with [Au] = 13.5 mg/liter (D) and [Au] = 127 mg/liter (E).
Fig. 2. A, B and C - Appearance of polysyloxane dyed with Pyrromelhene 597-8C9 without Au-NPs (A) and with Au-NPs at concentrations of [Au] = 13.5 mg/liter (B) and 127 mg/liter (C), D-F — dyed Au-NPs/polysyloxane composites ([Au] = 13.5 mg/liter), containing polymethine dye I (D), Lumogen Orange (dye V) (E) and Lumogen Red (Dye VI) (F).
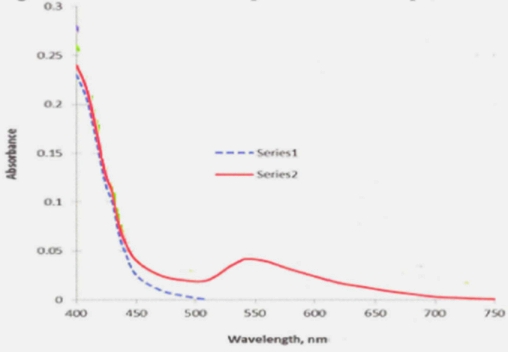
RESULTS AND DISCUSSIONFig.3 shows the absorbance spectra of Au nanoparticles in PSi.
Fig.3. Absorbance spectra of clear Psi (Series I) and Au-NPs/polysyloxane composites with concentration of Au-NPs 13.3 mg/liter (Series 2).
Yellowish color of PSi containing HAuCl4 (Series 1) is changing to grey- ruby color with formation of Au nanoparticles. This process could be detected by appearance of broad structure-less absorbance band at 540-545nm (Series 2).Fig.4 shows the fluorescence spectrum of Au-NPs/polysyloxane composites with concentration of Au-NPs 13.5 mg/liter
Fig.4. Fluorescence spectra of nanocomposite of Au-NPs/polysyloxane composites
with concentration of Au-NPs 13.5 mg/liter(excitation at 540 nm).
It was found (Fig.4) that the intensity of fluorescence band of Au/NPs in PSi at 575-580 nm grows with increasing Au/NPs concentration. At concentration of Au/NPs of 13.5 mg/liter and higher an additional broad band with maximum at 750-760 nm could be observed. These finding are in a good agreement with a literature data [11] related to interpretation of Au/NPs’ fluorescent properties. According to [11], at the first step, electrons are excited from the d-bands below the Fermi level to the sp - band above the Fermi level. After that electrons relax to the Fermi level via a variety of radiationless processes as well as holes scatter within the d-bands to match the positions of electrons in the momentum space. Finally, vertical recombination transitions terminating at the two bands d1 and d2 generate luminescence in the yellow-green and violet ranges of the spectrum. The predicted emission maxima correspond to 563 nm and 410 nm.with concentration of Au-NPs 13.5 mg/liter(excitation at 540 nm).
In our experiments we have observed only slightly red shifted fluorescence of first type, and emission around 410 nm was, probably absorbed by which was present in Au/NPs —PSi — nanocomposite.
Table 1 summarizes data on the positions of absorbance and fluorescence maxima of dyes I-VI in PSi with and without Au-NPs. In our previous investigation [9] it was found that due to a limited solubility of dyes 1 and II in PSi the absorbance value of saturated solutions of these dyes were below 0.07-0.08 (when the 1st method of making dyed Au-NPs/polysyloxane composites was used).
Table 1. Positions of absorbance and fluorescence maxima of dyes I-VI in PSi with and without Au-NPs.
Fig.5. Absorbance (series 1) and fluorescence spectra of dyes I and II in PSi
in absence of Au-NPs (Series 2) and in presence of Au-NPs at concentration of 13.5 mg/liter (Series 3).
However, as it is shown in Fig. 5, when 2nd method of making dyed Au- N Ps/polysyloxane composites was used much higher concentration of dyes I and II can be introduced in PSi. Spectral data on absorbance and fluorescence of these dyes without addition of Au-NPs in Psi seem to be in a good agreement with published results related to the spectral characteristics of these dyes in liquid solutions [12].in absence of Au-NPs (Series 2) and in presence of Au-NPs at concentration of 13.5 mg/liter (Series 3).
According to our previous investigation [9] performed on four polymethine dyes in PSi samples containing Au-NPs and without them all polymer compositions may be divided into 3 following groups:
A) Samples w here the Au-NPs increase the intensity of fluorescence for corresponding dyes.
B) Samples where the Au-NPs don’t affect the intensity of fluorescence for corresponding dyes or position of emission maximum.
C) Samples where the Au-NPs quench the emission at relatively short wavelength, with or without enhancing the emission at long wavelength
According to the literature data [10], generally, Au-NPs are believed to enhance the emission efficiency of dye molecules, acting as nano-antennae. In this case, through the resonance energy' transfer process, the dye absorption is improved and, in turn, the emission is also improved. However, the realization of such a system is not trivial and requires a close proximity' of the dye molecule to the Au-NPs, to the extent that the boundary gold atoms can be seen as ’grafted’ to the organic molecule. Besides, the certain ratio between the concentration of the Au-NPs and dye molecules seems to be important for the resonance energy transfer process.
This molecular architecture was previously successfully carried out through Au-NPs and the Rhodamine 6G molecule, exploiting the electrostatic attraction between the cationic form of the dye and the negative charge lying on the surface of the nanoparticles [8]. Results of this work, actually, confirmed an observation of authors [10] that perylenediimide dyes (dye V and VI) don’t establish coordination with the gold atoms which is proved by the lack of enhancement of emission intensity in the bulk samples.
Probably due to similarity between the structures of cationic forms of dye III (Rhodamine B) and Rhodamine 6C we observed some increase in fluorescence intensity after addition of Au-NPs to the solid solution of dye III in PSi. Similar effect has been noticed for dye I, however, the reason for such an enhancement requires a special investigation.
References
1. M. Westphalen, (J. Kreibig, J. Rostalski, H. Lfith, D. Meissner, Sol. Energy Mater. Sol. Cells 61 (2000) 97.
2.D. Oerkacs, S.H. Lim, P. Mathen, W. Mar, E.T. Yu, Appl. Phys. Lett. 89 (2006) 093103.
3.R.B. Konda, R. Mundle, H. Mustafa, O. Bamiduro. A.K. Pradhan, U.N.Roy, Y. Cui, A. Burger, Appl. Phys. Lett. 91 (2007) 191111.
4.AJ. Morfa, K.L. Rowlen, T.H. Reilly UI, MJ. Romero, J. Van de Lagemaat, Appl. Phys. Lett. 92 (2008) 13504.
5.S.-S. Kim, S.-1. Na, J. Jo, D.-Y. Kim, Y.-C. Nah, Appl. Phvs. Lett. 93 (2008) 073307.
6.B.P. Rand, P. Peumans, S.R. Forrest, J. Appl. Phys. 96 (2004), 7519.
7.K. Kim, D.L. Carroll, Appl. Phys. Lett. 87 (2005) 203113.
8.M. Iosin, P. Baldeck, S. Astilean, Nuclear Instruments and Methods in Physics Research, Section B, 267 (2) (2009) 403-405.
9.N.N.Barashkov, et al. “Experimental study of absorbance and fluorescence spectra of colloidal dispersion of Au nanoparticles in solid solutions of organic dyes in polysiloxane”. Abstract of presentation on ACS National Meeting, Dallas, 2014.
10.M. Buffa, S. Carturan, M.G. Debije, A. Quaranta, G. Maggioni, Solar Energy Materials & Solar Cells, 103 (2012) 114—118.
11.M. A. Noginov, G. Zhu, V. I. Gavrilenko, OPTICS EXPRESS, v.15 (24), (2007), 15648.
12 G. P. Grabchuk, et al, Mol. Cryst. Liq. Cryst., v. 536 (2011), 130-139.
 Схожі теми
Схожі теми» SURFACE STRUCTURE OF BIOMIMETIC HYDROXYAPATITE NANOPARTICLES
» MOLECULAR STRUCTURE, QUANTUM-CHEMICAL INVESTIGATION AND SPECTRAL PROPERTIES HALOGEN CONTAINED DIMERS OF BENZENE
» USING QUANTUM DOTS IN THE CREATION OF POLYMER FLUORESCENT SOLAR CONCENTRATORS
» PREPARATION AND PROPERTIES OF THERMOPLASTIC FLUORESCENT PIGMENTS BASED ON ALIPHATIC POLYUREAS AND COPOLY(UREA)URETHANES WITH FRAGMENTS OF AROMATIC SULFONAMIDE IN POLYMER CHAIN
» STUDY OF FREE FORMALDEHYDE REDUCTION AND FIXATION OF GASEOUS FORMALDEHYDE AS BY-PRODUCT OF OBTAINING OF FLUORESCENT PIGMENTS BASED ON AMINE-SULFONAMIDE-FORMALDEHYDE RESINS
» MOLECULAR STRUCTURE, QUANTUM-CHEMICAL INVESTIGATION AND SPECTRAL PROPERTIES HALOGEN CONTAINED DIMERS OF BENZENE
» USING QUANTUM DOTS IN THE CREATION OF POLYMER FLUORESCENT SOLAR CONCENTRATORS
» PREPARATION AND PROPERTIES OF THERMOPLASTIC FLUORESCENT PIGMENTS BASED ON ALIPHATIC POLYUREAS AND COPOLY(UREA)URETHANES WITH FRAGMENTS OF AROMATIC SULFONAMIDE IN POLYMER CHAIN
» STUDY OF FREE FORMALDEHYDE REDUCTION AND FIXATION OF GASEOUS FORMALDEHYDE AS BY-PRODUCT OF OBTAINING OF FLUORESCENT PIGMENTS BASED ON AMINE-SULFONAMIDE-FORMALDEHYDE RESINS
СУЧАСНЕ МАТЕРІАЛОЗНАВСТВО ТА ТОВАРОЗНАВСТВО: ТЕОРІЯ, ПРАКТИКА, ОСВІТА :: Актуальні питання наукового та практичного матеріалознавства.
Сторінка 1 з 1
Права доступу до цього форуму
Ви не можете відповідати на теми у цьому форумі